Light associated with saltwater primarily manifests through biological processes or can be facilitated by saltwater's chemical properties in engineered systems. These phenomena differ significantly in their origin and nature.
Bioluminescence: Nature's Glow in Saltwater
The most prominent form of light from saltwater is bioluminescence. This is the production and emission of light by living marine organisms through a chemical reaction.
- Key Organisms: This phenomenon is common in various marine life, including dinoflagellates (phytoplankton like Noctiluca scintillans, often responsible for "glowing tides"), certain bacteria, jellyfish, ctenophores (comb jellies), crustaceans, and some fish.
- Chemical Basis: Bioluminescence typically involves a substrate called luciferin and an enzyme called luciferase. The oxidation of luciferin, catalyzed by luciferase, releases energy in the form of light. The specific chemicals can vary between species, leading to different light colors, most commonly blue or green, as these wavelengths transmit best through water.
- Triggers and Functions: Light emission is often triggered by mechanical stimulation, such as wave action, the movement of a boat, or a predator. The functions of bioluminescence are diverse, including defense (startling predators, "burglar alarm" effect), attracting prey, camouflage (counter-illumination), and communication for mating.
Saltwater as an Electrolyte for Light Generation
Saltwater can also be instrumental in producing light indirectly by serving as an electrolyte in an electrochemical cell, commonly known as a saltwater battery. In this scenario, the saltwater itself does not emit light but enables the generation of electricity to power a light source.
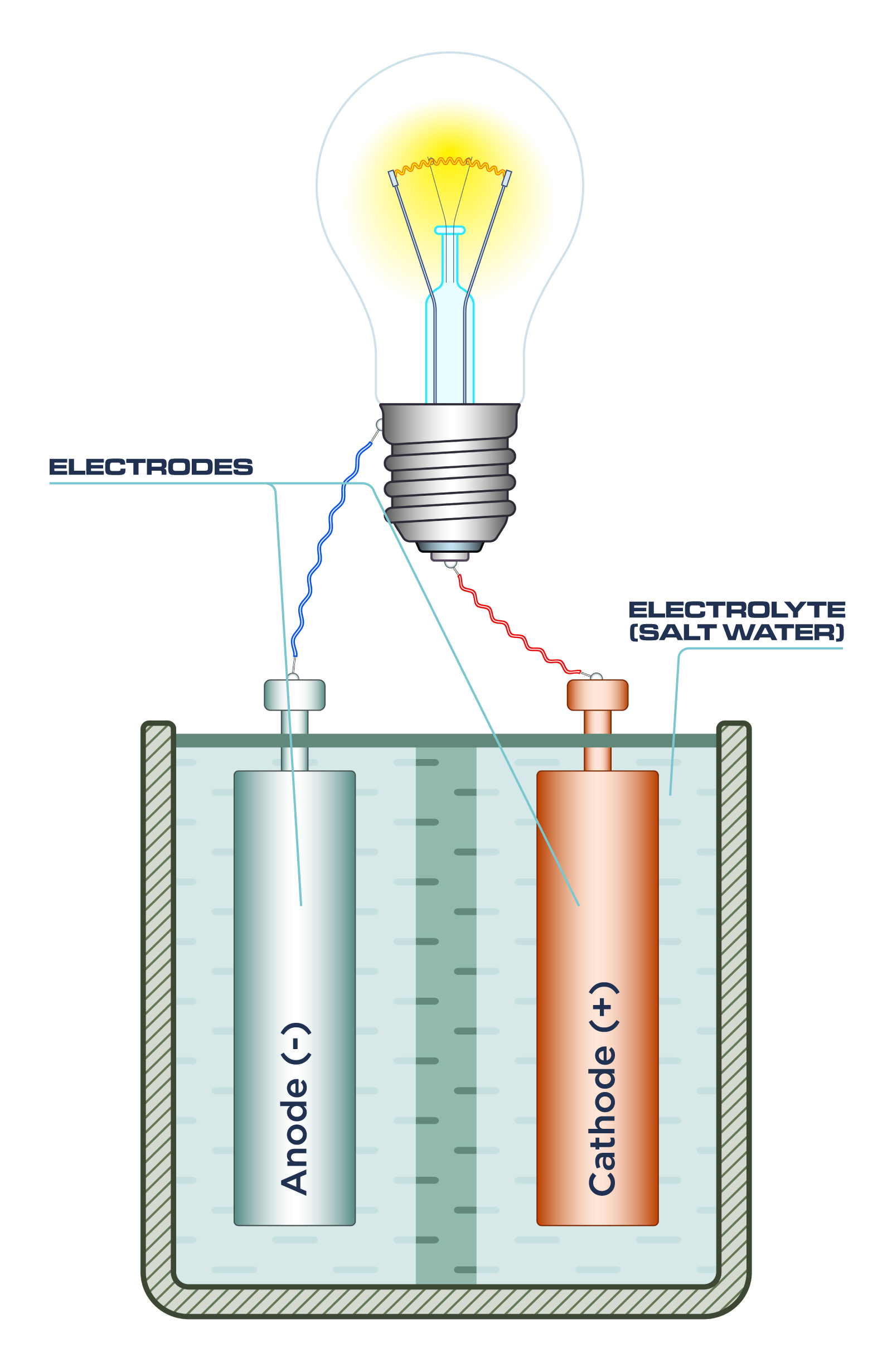
Dissolved salts (e.g., sodium chloride, NaCl) in water dissociate into ions (Na+ and Cl-). These mobile ions allow for the conduction of electric current between two dissimilar metal electrodes (e.g., copper and zinc) immersed in the saltwater. This creates a potential difference, and the resulting electrical energy can be used to power a low-energy light, such as an LED. Such devices are sometimes used in emergency lighting or educational demonstrations, showcasing basic electrochemical principles.